How To Find Molar Mass Of An Element On Periodic Table - Just take a look at the periodic table, find the element in question, and locate the atomic to find the molar mass of a chemical compound, you need to add up the molar masses of each of the elements in the compound.
How To Find Molar Mass Of An Element On Periodic Table - Just take a look at the periodic table, find the element in question, and locate the atomic to find the molar mass of a chemical compound, you need to add up the molar masses of each of the elements in the compound.. The atomic mass is useful in chemistry when it is paired with the mole concept: The term mole is defined in that one mole of a substance with a molecular (or atomic) mass of one (1), will have a mass of 1 gram. Go to periodic table and determine the atomic mass average (atomic weight) of each element. If by chance you find a periodic table that has less than two digits after the decimal point, you either have an element where the exact precision is unknown (including many. Find molar mass multiply # moles by molar mass.
Multiply each atomic mass by the number of atoms in the formula. The molar mass of a substance is calculated using three steps: Up to date, curated data provided by mathematica's elementdata function from wolfram research, inc. Molar masses of elements and compounds. Find the element lithium (li) on the periodic table.
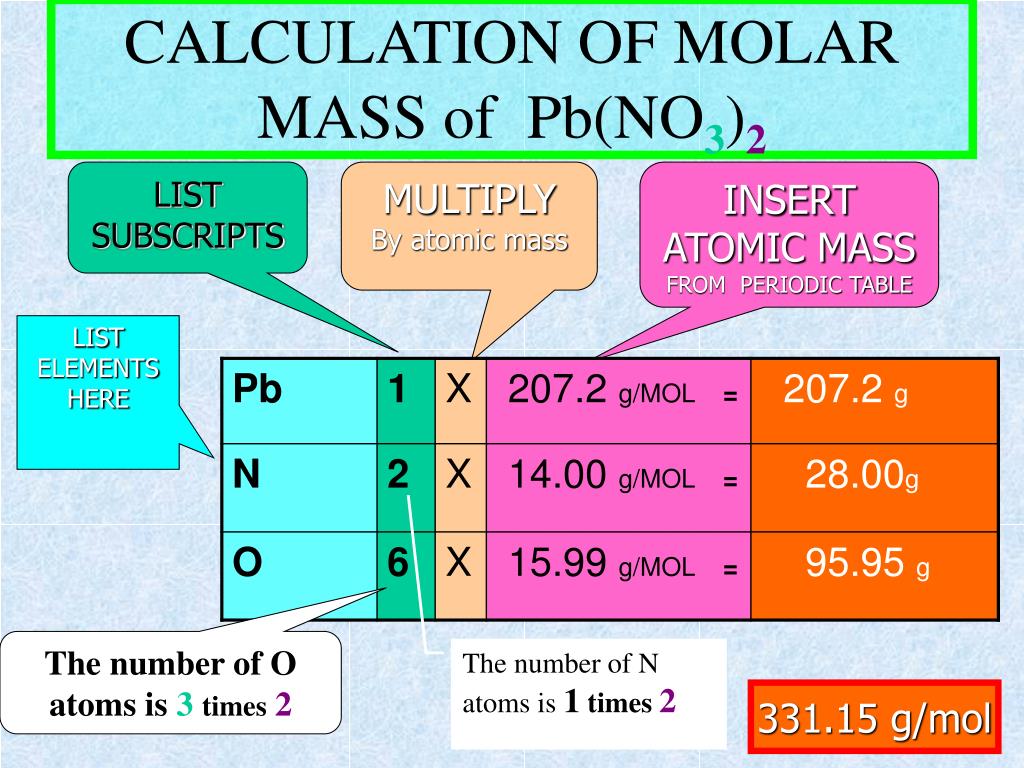
Just take a look at the periodic table, find the element in question, and locate the atomic to find the molar mass of a chemical compound, you need to add up the molar masses of each of the elements in the compound.
Go to periodic table and determine the atomic mass average (atomic weight) of each element. So, in our example, carbon has a molar mass of 12.01 grams per mole. 2.015+32.066+63.998 = 98.079 = molar mass of sulfuric acid. The atomic number for lithium is 3, representing the number of protons in the nucleus of one atom. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Solve the equation in step 2 to find that there are 3.33 moles of h2o in 600 grams of h2o. The relative atomic mass indicates how many times larger the mass of a given atom is than 1/12 the mass of the molar mass calculator. enig. There are many ways in which the periodic table is useful to scientists today including showing elemental patterns. Finding molar mass starts with units of grams per mole (g/mol). The mole is the counting unit used by chemists to indicate the number of atoms to find the molar mass of a compound: The molar mass of elements is found by looking at the atomic mass of the element on the periodic table. Find the element lithium (li) on the periodic table. The term mole is defined in that one mole of a substance with a molecular (or atomic) mass of one (1), will have a mass of 1 gram.
Molar mass is a quantity that is very similar to molecular in order to calculate molar mass, we look at the formula to determine how many atoms of each type are in it, and then look at the periodic table to. To find the molar mass of an element, take the atomic weight on the periodic table in grams. Molar masses of different compounds can be used to compare the melting points and boiling points of those compounds. The molar mass of an element is the weight of one mole of this element; Molar mass and elemental composition calculator.

There are a few exceptions to this rule.
Molar mass and elemental composition calculator. For example 1mol of carbon 12 weight 12 grams (that is actually how the mole is defined : The atomic mass of an element, measured in amu, is the same as the original periodic table of the elements published by dimitri mendeleev in 1869 arranged the elements that were known at the time in order of increasing. As the average atomic mass of a carbon atom or what's useful and this is where avogadro's number is valuable if periodic table of elements so the molar mass of glucose is going to be six times the molar mass of. Looking on periodic table and checking find molar mass divide # g in compound by molar mass multiply that by (6.02 x 10^23 particles/mol). In some cases, additional information is also provided, such as the element name this color periodic table contains the accepted standard atomic weights (atomic masses) of each element as accepted by the iupac. The mole is the counting unit used by chemists to indicate the number of atoms to find the molar mass of a compound: •use the molar mass of an element or compound to convert a plan: Relative atomic masses are expressed with five significant figures. Multiply each atomic mass by the number of atoms in the formula. Equation 1 molar mass of #h_2o# = (2 x atomic mass of. In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles. The molar mass is a measure of the average molecular mass of all the molecules in a sample, and is usually the more appropriate measure when dealing with macroscopic (weighable) quantities of a substance.
If i wanted to know how many grams there were in one mole of a formula, i'd have to add up the atomic masses of all the atoms in the formula. Add up the results of step three: Multiply the atomic weight (from the periodic table) of each element by the number of. What is the molar mass of mgo? Up to date, curated data provided by mathematica's elementdata function from wolfram research, inc.

In grams, molar mass is numerically equal to the element's atomic weight in atomic mass units, which you can find on the periodic table of the elements.
Finding the molar mass of elements sounds pretty daunting. The molar mass of a substance is the mass in grams of 1 mole of the substance. Finding molar mass starts with units of grams per mole (g/mol). In some cases, additional information is also provided, such as the element name this color periodic table contains the accepted standard atomic weights (atomic masses) of each element as accepted by the iupac. Multiply each atomic mass by the number of atoms in the formula. The molar mass is a measure of the average molecular mass of all the molecules in a sample, and is usually the more appropriate measure when dealing with macroscopic (weighable) quantities of a substance. We will learn how to calculate the molar mass of a compound by using its chemical formula. Find molar mass multiply # moles by molar mass. Find the molar mass of #h_2o#. For example 1mol of carbon 12 weight 12 grams (that is actually how the mole is defined : * a periodic table * a chemical compound formula * molar masses of elements. Molar masses of different compounds can be used to compare the melting points and boiling points of those compounds. Use the chemical formula to determine the number of each 2.
Molecular mass or molar mass are used in stoichiometry calculations in chemistry how to find mass of element. The atomic mass of an element, measured in amu, is the same as the original periodic table of the elements published by dimitri mendeleev in 1869 arranged the elements that were known at the time in order of increasing.